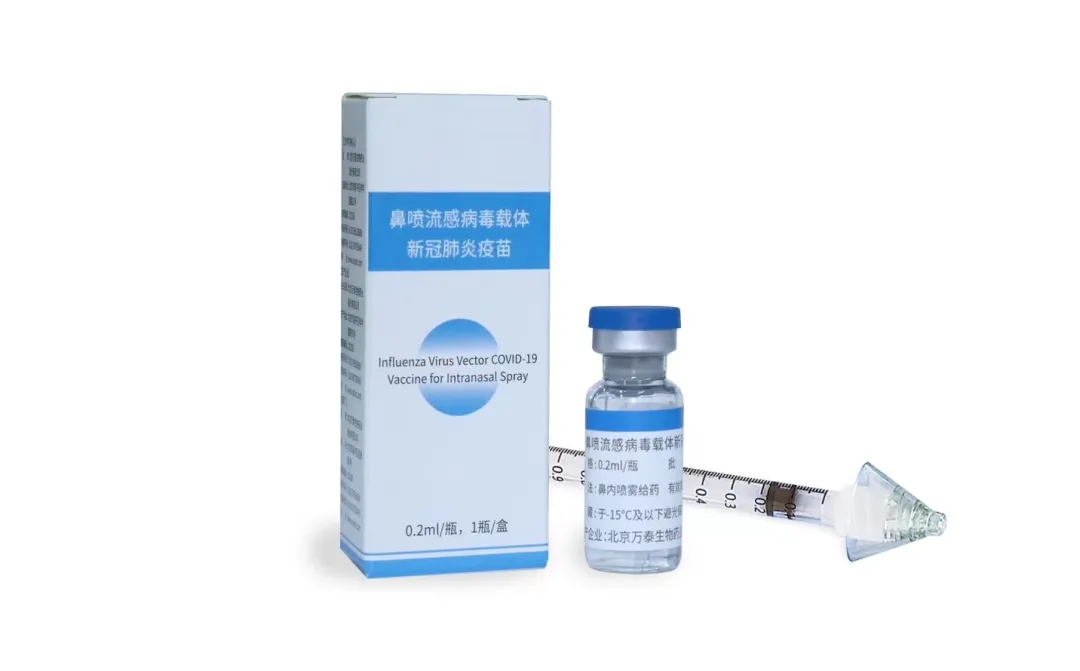
Pneucolin® Intranasal vaccine
In rapid response to the outbreak of the novel coronavirus, SARS-CoV-2, the HKU Microbiology team quickly developed an influenza-based COVID-19 vaccine (DeINS1-SARS-CoV-2-RBD) targeting the SARS-CoV-2 receptor-binding domain (RBD) in February 2020.
In March 2020, Tencent Foundation donated HKD five millions to the vaccine project. Other fundings have come from the Health and Medical Research Fund (HMRF) of the HKSAR and the Coalition of Epidemic Preparedness Initiative (CEPI). Developed by HKU in collaboration with Xiamen University and Wantai Biopharm, the intranasal vaccine, Pneucolin®, successfully passed phase one to three clinical trials in Mainland China, Hong Kong SAR, and in four countries, i.e. South Africa, Philippines, Columbia and Vietnam.
Clinical trial data have shown that Pneucolin can significantly reduce symptomatic infections, prevent hospitalization and death caused by COVID-19. In December 2022, Pneucolin was approved for emergency use in humans in Mainland China, becoming the first intranasal vaccine for COVID-19 that received such approval. It has also been recommended as one of the vaccines for booster in response to the COVID-19 epidemic. As of Nov 2023, Pneucolin has been used in Beijing, Xiamen, Yancheng, Quanzhou, Fuzhou, and Suzhou.
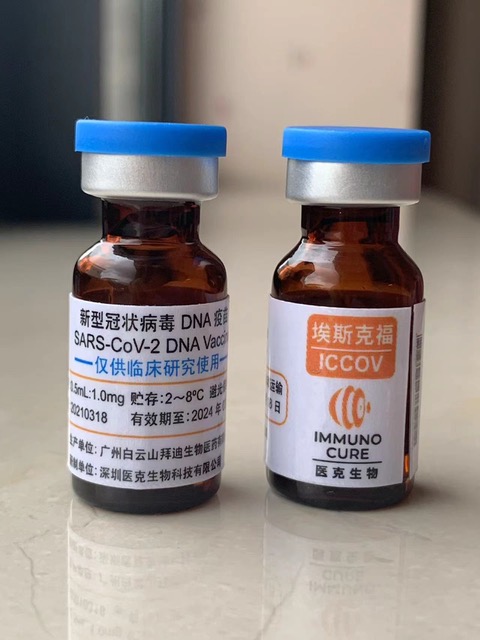
ICVAX and ICCOV
Both ICVAX and ICCOV were invented by our HKU team and licensed to Immune Cure.
ICVAX, a PD-1-enhanced therapeutic HIV DNA vaccine, has been successfully validated in mouse and non-human primate models for enhanced immunogenicity and protective efficacy. As a DNA vaccine, ICVAX can be repeatedly used to boost anti-HIV immune responses without issues of pre-existing immunity against vaccine vector. Its safety has also been demonstrated in preclinical GLP toxicity studies. Immuno Cure has already obtained the IND approval from the National Medical Products Administration (NMPA) for conducting a Phase 1 clinical trial of IMC-001 at the Shenzhen Third People’s Hospital in mainland China.
Based on the same PD-1-enhanced DNA vaccine platform, a COVID-19 DNA vaccine for SARS-CoV-2, namely ICCOV, has been constructed by HKU team. ICCOV not only primes a high level of neutralizing antibody response against SARS-CoV-2, but also induces a potent cytotoxic CD8+ T cell immune responses with demonstrated protection in mouse models. Critically, the ICCOV prime and intranasal DeINS1-SARS-CoV-2-RBD boost vaccine strategy conferred nasal prevention of SARS-CoV-2 infection, which has not been found with mRNA and inactivated vaccines. The first-in-human clinical trial of ICCOV has recently completed in Hong Kong. The vaccine is safe and immunogenic in humans. It is useful as a booster vaccine targeting SARS-CoV-2 variants.
We have established drug screening platforms for the discovery of novel antimicrobials. Many of these antimicrobials are broad-spectrum and can be used for future emerging infection challenges. In order to identify effective therapeutic and vaccine strategies, we have established representative animal (hamster, rat) and next-generation culture models (organoids).
We set up the in-silico and in-vitro
high-throughput drug discovery platforms using high-performance computers and
automatic screening robotics, which is a highly cost-effective approach.
These
world-class platforms have allowed us to rapidly identify various potent
antivirals, including nucleozin, AM580 and clofazimine. Some of them have
been used in clinical trials to treat patients. The physiologically-relevant
organoid models can be extensively utilized in biomedical research and
translational applications, including disease modeling, therapeutic
development, toxicological evaluation, and preclinical medicine, etc. It also overcomes the existing
limitations of cancerous cell culture models, particularly their different
physiology and cell biology than normal human cells.
We
established the world’s first hamster model as cornerstones for studying the
transmission, pathogenesis, therapeutics, and vaccines of COVID-19. These
world-leading translational innovations have made long-lasting policy-changing
impact on the study infectious diseases. They have also enhanced academia-industry
collaborations in the Greater Bay Area with several patents and seed companies, highlighting HKSAR as an international hub and leader in infectious disease
research innovations.


created with
WordPress Website Builder .