Dr Jane Zhou Jie
Assistant Professor
Department of Microbiology
I did
my PhD research in the Department of Microbiology at The University of Hong
Kong in 2003-07 under the supervision of Prof Zheng Bojian and Prof Yuen
Kwok-yung. After my postdoctoral training at the University of California, San
Francisco (UCSF), I joined the department in 2009 as a research assistant professor. During the past two decades, I have focused on the
pathogenesis of viral infections, particularly those from emerging respiratory
viruses. To explore the host vulnerability to pandemic influenza virus H1N1 and
avian influenza virus H7N9, we integrated genome-wide disease association data
and gene expression profiles to prioritize candidate genes for functional
characterization, and we identified a number of disease-important genes for
human influenza, including CD55, Galectin 1, TMPRSS2, GLDC and SPINK6.
Conventional
biomedical research relies upon the use of experimental models such as cell
lines and animal models. Human tissue such as the respiratory tract, however,
are composed of diversified and specialized cell types. Cell lines
and animals are unable to recapitulate the high complexity and specific
features of human tissue. Recent advances in stem-cell technology enable
the generation of organoids, also known as “mini-organ or organ-in-a-dish”. These
are 3D cellular clusters that can faithfully mimic the architecture and
functionality of their native organ, thus representing a major breakthrough in
human biology.
Organoids
enable scientists to recapitulate and study in vivo biological processes
in culture plates, opening a new arena for diverse biomedical and
pharmaceutical applications. There are multiple types of organoids in the
rapidly growing market, human intestinal organoids and patient-derived cancer
organoids account for the biggest proportion.
Yet,
a robust and well-defined respiratory organoid culture system is elusive. The
human respiratory epithelium, the primary infection site of respiratory
pathogens including SARS-CoV-2, is lined with two distinct types of epithelia,
the airway and the alveolar epithelium. Immortalized cell lines such as A549 or
Calu3 are commonly utilized to study respiratory biology and pathology. These
homogeneous cell lines, however, are unable to simulate the multicellular
complexity and functional diversity of human respiratory epithelia, let alone
model respiratory infections, including SARS-CoV-2 infection.
Our team established a two-phase, bipotential organoid culture system that allows scientists to reconstruct and expand the entire human respiratory epithelium in culture plates, which is the first and most advanced respiratory organoid culture system in the world. We derive organoids from primary lung tissues and nasal cells in a highly efficient manner, which provides a stable and self-renewable source for long-term expansion. We induce proximal and distal differentiation in the long-term expandable organoids and generate mature airway and alveolar organoids that morphologically and functionally phenocopy the native human airway and alveolar epithelium.[1]
[2]
[3] Specifically, We establish a bipotential organoid culture system able to rebuild and expand the entire human respiratory epithelium in vitro with high efficiency and stability. These physiologically-active respiratory organoids are optimal in vitro models for studying respiratory diseases including influenza and COVID-19.
Respiratory organoid culture system established by HKU Microbiology
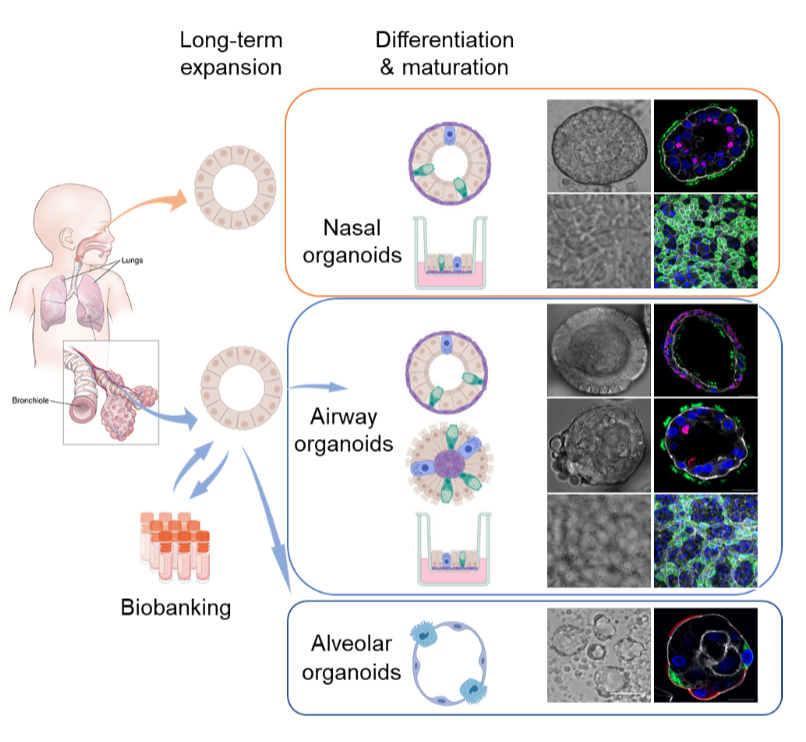
In the wake of the COVID-19 outbreak,
we demonstrated SARS-CoV-2 replication in human intestinal organoids,
suggesting that the human gastrointestinal tract is an alternative viral
transmission route. We also developed the first bat intestinal organoid
culture. Bat organoids are fully susceptible to SARS-CoV-2, lending support to
the proposed bat origin.[4] Importantly, bat organoid opens up a new avenue to isolate and cultivate the
previously uncultivable viruses and to assess the zoonotic potential.[5]
Apart from the vast applications in
basic biology and translational research, these world-leading respiratory and
intestinal organoids are biologically-relevant tools for recapitulating and
evaluating the infectivity of emerging viruses to humans, a longstanding threat
to public health worldwide. Nature and a subsidiary journal recently
reported our organoid-based virus research, especially my research work on
COVID-19.[6]
[7] The organoid models established by our team can be extensively utilized in multiple
disciplines for studying human biology and pathology beyond our research area
of virus-host interaction.
[1] Zhou J, Li C, Sachs N, Chiu MC, Wong BH, Chu
H, Poon VK, Wang D, Zhao X, Wen L, Song W, Yuan S, Wong KK, Chan JF, To KK,
Chen H, Clevers H, Yuen KY. Differentiated human airway organoids to assess
infectivity of emerging influenza virus. Proceedings
of the National Academy of Sciences U S A. 2018 Jun
26;115(26):6822-6827.
[2] Chiu MC, Cun Li, Xiaojuan Liu, Yifei Yu, Jingjing Huang, Zhixin Wan,
Ding Xiao, Hin Chu, Jian-Piao Cai, Biao Zhou, Ko-Yung Sit, Wing-Kuk Au, Kenneth
Kak-Yuen Wong, Gang Li, Jasper Fuk-Woo Chan, Kelvin Kai-Wang To, Zhiwei Chen,
Shibo Jiang, Hans Clevers#, Kwok-Yung Yuen#, Zhou J#. 2022. A bipotential organoid model of respiratory epithelium
recapitulates high infectivity of SARS-CoV-2 Omicron variant. Cell Discovery.
2022 Jun 17;8(1):57.
[3] Chiu MC, Li C, Liu X, Song W, Wan Z, Yu Y, Huang J, Chu H, Cai J,
Kai-Wang To K, Yuen KY, Zhou J#.
Human nasal organoids model SARS-CoV-2 upper respiratory infection and
recapitulate the differential infectivity of emerging variants. mBio. 2022 Aug 30;13(4):e0194422
[4] Zhou
J#, Li C. Liu X, Chiu MC, Zhao X, Wang D, Wei Y, Lee A, Zhang AJ,
Chu H, Cai JP, Yip CC, Chan IHY, Wong KKY, Tsang OTY, Chan KH, Chan JF, To KK,
Chen H, Yuen KY#. Infection of bat and human intestinal organoids by
SARS-CoV-2. Nature Medicine. 2020 Jul;26(7):1077-1083.
[5] Liu X, Li C, Wan Z, Chiu MC, Huang J, Yu Y,
Cai JP, Chu H, Jiang S, Kwok-Yung Yuen#, Zhou J#. Analogous comparison unravels heightened
antiviral defense in bat organoids and boosted viral infection upon
immunosuppression. Signal Transduction & Targeted Therapy. 2022 Dec
19;7(1):392.
[6]Nature. 2021 May;593(7860):492-494. https://www.nature.com/articles/d41586-021-01395-z
[7]Nature Reviews Molecular Cellular
Biology. 2020 Jul;21(7):355-356. https://www.nature.com/articles/s41580-020-0258-4
created with
WordPress Website Builder .